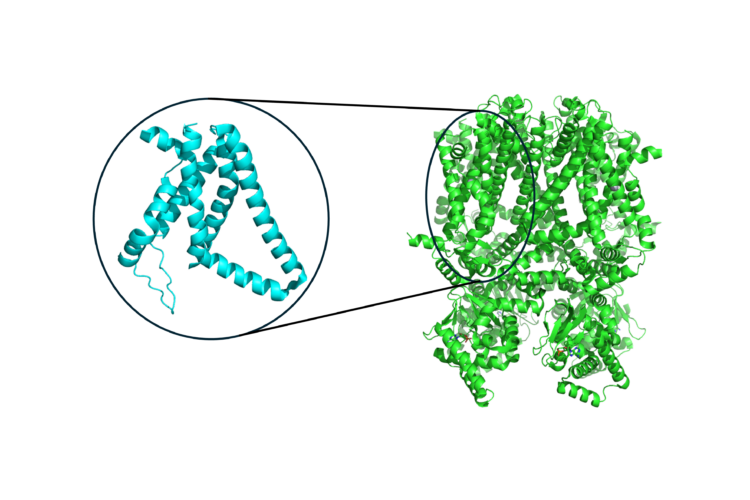From Protein Structures to Clean Energy Materials to Cancer Therapies: Using AI to Understand and Exploit X-ray Damage Effects
X-rays are not just the workhorse for imaging bone structures in our bodies, they are also the workhorse of materials characterization techniques across the biological, chemical and physical sciences. However, the absorption of x-rays by atoms creates unstable states which undergo complex decay process that can damage the structures during their characterization.
Interestingly though, this x-ray damage process is not all bad. In some cases, detecting it can itself be a powerful characterization tool; and furthermore, the effect is now being explored in novel cancer therapies that selectively target tumor cells. The physics underlying damage effect is described by quantum mechanics (the theory describing the behavior of atoms and electrons), this limits its effective simulation in complex real-world systems. However, the opportunity for AI accelerated predictions of more complex systems has emerged through the development of accurate quantum mechanical methods for generating small molecule datasets and the availability of benchmark experiments.
X-rays can characterize the arrangement of atoms forming the shape of complex materials, such as proteins or nanoparticle catalysts through x-ray scattering techniques. They can also interrogate the arrangement of electrons forming chemical bonds around a selected atom site through x-ray spectroscopy, which involves the absorption of x-rays by the atoms. Whilst the dosage of x-rays safely used in everyday medical applications is relatively low; effectively interrogating the microscopic world of atoms requires exceptionally bright x-ray sources generated at large-scale facilities with circular particle accelerators called synchrotrons. Synchrotrons pass electrons through a sequence of magnets at almost the speed of light, where they can then produce x-rays 10 billion times brighter than the sun. Researchers utilize the powerful characterization properties of the bright x-rays on subjects ranging from combustion engines, batteries, protein structures, cancer treatments and advanced research materials.
X-ray damage effects happen when x-ray absorption interferes with x-ray scattering measurements. For example, in proteins that contain metal atoms, the absorption of metal atoms by the x-rays dominates the interaction during the scattering measurement. Consequently, almost all such protein structures reported in the protein databank are compromised. This happens because the x-ray absorption removes the electrons most tightly bound to the atoms nucleus. This creates an unstable state that collapses by a complicated decay process which ejects multiple electrons from the atom. The removal of negatively charged electrons leaves a positively charged environment about the metal atom which changes the protein structure during the x-ray scattering characterization.
However, this x-ray damage process can in-fact be utilized. Detecting these ejected electrons provides a complex but detailed characterization of the electronic arrangements about the absorbing atom site, which offers the opportunity for more sensitive x-ray spectroscopy methods. This is because the ejected electrons are not tightly bound to the nucleus and are more involved with the bonding to the neighboring atoms. Another useful property of the ejected electrons is that they can have relatively low energies compared to fluorescence decay and the nuclear decay of radioactive atoms. The low energy ejected electrons have short travel distances in aqueous environments because they are likely to ionize nearby water molecules. This makes them effective at characterizing the structure of a materials surface in an aqueous solution. This has successfully been applied to characterizing the differences in water molecule arrangements at the material surface and bulk solution in a battery cell system.
The short travel distances in aqueous environments has applications beyond materials characterization. Novel radionucleotide cancer therapies also exploit this effect. Once a radionucleotide atom in a molecule undergoes a nuclear decay process, a tightly bound electron is also lost from it. This mimics x-ray absorption and initiates the same decay cascade process. Near a tumor cell, the low energy electrons ejected from the radionucleotide and neighboring atoms can effectively damage the tumor whilst causing minimal damage to the surrounding tissue. This treatment is therefore less damaging to the patient than traditional cancer therapies using the high energy alpha and beta decays.
Despite all these advantages, it’s hard to simulate the decay process as it relies on quantum mechanics. Furthermore, the number of possible decay channels scales exponentially with the size of the system and simulations are thus restricted to atoms, small molecules, and simplistic aqueous environments.
However, reliable AI predictions of larger systems are now possible through recent developments in accurate and efficient methods for small (5-10 atoms) organic molecules, and the availability of benchmark experimental data on these systems and more complex ones. We are developing a deep neural network that inputs information of the atomic placements about the absorbing atom site and outputs the decay signal. The input only requires the knowledge of the molecular geometry, and the trained model will therefore bypass the need for further quantum mechanics calculations.

Because the decay of each individual absorbing atom is mostly affected by its adjacent atoms, we believe our trained model will accurately predict the decay of organic molecules too large for a quantum mechanics simulation. Our model will be trained across thousands of different atomic environments across a set of mid-size organic molecules. Therefore, the model should be able to combine any number of different local atomic environments together to generate the decay signals of much larger systems. Successfully demonstrating this will open a new AI research direction, where the continual development of dataset diversity, model architecture and experimental collaboration will pave the way for AI to drive understanding and exploit the role of this x-ray damage effect in important real-world applications.
This work was funded by the Eric and Wendy Schmidt AI in Science Postdoctoral Fellowship, a Program of Schmidt Futures.

Expanding Our Vocabulary of Vision Using AI
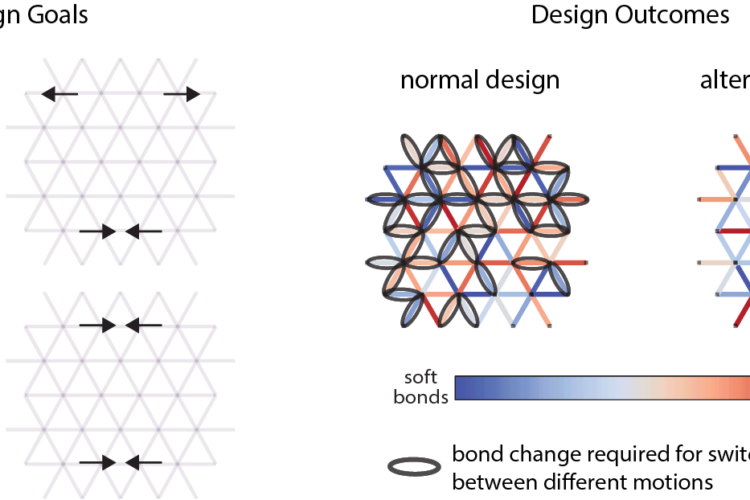
Teaching materials to adapt
Towards New Physics at Future Colliders: Machine Learning Optimized Detector and Accelerator Design