Automated Material Discovery for More Sustainable Plastics: Describing Polymer Chemistry for Human and Machine
Plastics are a double-edged sword for our environment. Every year, about 500 million tons of plastic are produced, most of which originate from petrochemical sources and end up as waste. Not only does this immense volume of plastic not decompose for centuries, but it also frequently escapes proper disposal, leading to environmental pollution. A stark example is the Great Pacific Garbage Patch, a massive accumulation of plastic in the Pacific Ocean. To put this pollution in perspective, if this patch were made entirely of typical 10-gram shopping bags, it would amount to approximately 10 billion bags, exceeding the human population on Earth.
However, it’s crucial to recognize that not all plastics are inherently harmful. They are incredibly versatile and economical, finding use in everything from everyday clothing and packaging to essential roles in micro-electronics, medical devices, and even battery electrolytes. Our goal, therefore, isn’t to eliminate plastics altogether but to explore sustainable alternatives and rethink our reliance on single-use items.
Sustainable Solutions
The path forward involves making plastics more sustainable. This could be achieved by using plant-based materials, ensuring compostability, or improving recyclability. Significant scientific progress has been made in developing such materials. However, the challenge doesn’t end with sustainability; these new materials must also functionally outperform their predecessors. A notable example is the case of Sunchips’ compostable bags, which, despite being environmentally friendly, were rejected by consumers due to their loud crinkling sound. This illustrates the need for sustainable plastics to meet both environmental and functional standards.
To address this challenge, science is rapidly advancing in the exploration of new materials through automated experimentation, computer simulations, and machine learning. However, these methods require a universal language to describe polymeric materials understandable to both computers and human scientists.
This brings us to the core of what makes plastics unique: polymers. Derived from the Greek words ‘πολύς’ (many) and ‘μέρος’ (parts), polymers are long molecules composed of repeating units called monomers. For instance, simplified polyethylene, the material of common shopping bags, is essentially a long chain of carbon atoms.
Visualizing the structure of a polymer like polyethylene can be straightforward for someone with a chemistry background. A basic representation using text characters, with dashes indicating covalent bonds between carbon (C) and hydrogen (H) atoms, looks like this:
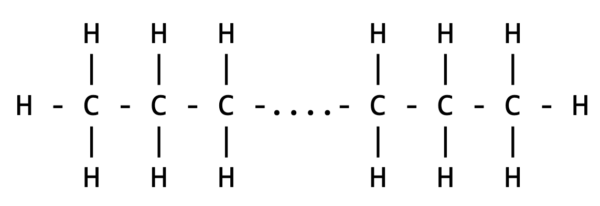
This representation, while instructive for humans, poses a challenge for computers, especially due to its two-dimensional nature. By simplifying the notation and assuming implicit hydrogen bonds, we can transform it into a one-dimensional string more comprehensible to computers:
CCC….CCC
From SMILES Notation to BigSMILES
Expanding this concept leads us to SMILES (Simplified Molecular Input Line Entry System), a widely-used notation for small molecules. However, traditional SMILES doesn’t address the varying lengths of polymers, as a real polymer chain consists of thousands of carbon atoms. Writing them all out would be impractical and overwhelming.
This challenge is elegantly solved by a notation specifically designed for polymers, known as BigSMILES. It represents the repeating nature of monomers in a compact and understandable form. For instance, a simplified version of polyethylene can be represented as:
{[] [$]CC[$] []}
This format not only makes it easier for humans and machines to interpret but also allows for more detailed specification of connections and types of monomers, reflecting a wide range of realistic polymeric materials.
Representing Molecular Weight Distribution
One crucial aspect not yet addressed is the variation in the length of polymer chains in a material, known as the molecular weight distribution. This is where the generative version of BigSMILES, G-BigSMILES, comes into play. It allows the specification of molecular weight distributions, as demonstrated in the following example:
{[] [$]CC[$] []}|schulz_zimm(5000, 4500)|
Here, the Schulz-Zimm distribution is used to describe the average chain lengths in terms of molar masses (M_w and M_n). Here the molar mass is a description of how long the polymer chains are i.e. how many monomers are repeated to compose the chain molecule.
Closing the Loop: From Notation to Material Exploration
With G-BigSMILES, we can now comprehensively describe polymeric materials in a way that’s both human-readable and machine-processable. Our Python implementation allows for the generation of molecular models based on these descriptions, facilitating the exploration of material properties through computer simulations.
Real-world polymeric materials are often more complex, involving branched chains or multiple monomer types. For more in-depth examples, readers are encouraged to consult our publication and GitHub repository.
Looking Ahead: AI-Driven Material Discovery
The next step in our project involves enhancing the interpretability of G-BigSMILES for machines. By translating these notations into graph structures, we aim to enable AI algorithms to suggest new material compositions. The goal is to ensure that these suggestions are not only chemically valid but also optimized for performance, paving the way for more efficient and sustainable material discovery.
This work was funded by the Eric and Wendy Schmidt AI in Science Postdoctoral Fellowship, a Program of Schmidt Futures.
